Multiple Sclerosis, COVID-19 and Vaccines: Making the Point
Abstract
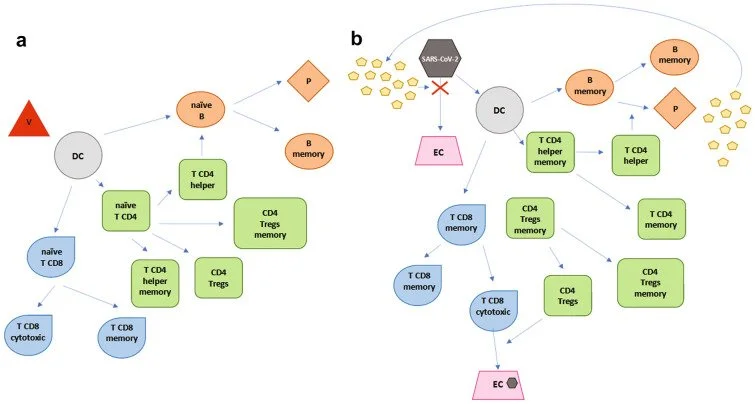
On 11 March 2020, the World Health Organization declared the coronavirus disease 19 (COVID-19) outbreak a pandemic. In this context, several studies and clinical trials have been conducted since then, and many are currently ongoing, leading to the development of several COVID-19 vaccines with different mechanisms of action. People affected by multiple sclerosis (MS) have been considered high-risk subjects in most countries and prioritized for COVID-19 vaccination. However, the management of MS during the COVID-19 pandemic has represented a new challenge for MS specialists, particularly because of the initial lack of guidelines and differing recommendations. Despite an initial hesitation in prescribing disease-modifying drugs (DMDs) in naïve and already treated patients with MS, most national neurology associations and organizations agree on not stopping treatment. However, care is needed especially for patients treated with immune-depleting drugs, which also require some attentions in programming vaccine administration. Many discoveries and new research results have accumulated in a short time on COVID-19, resulting in a need for summarizing the existing evidence on this topic. In this review, we describe the latest research results on the immunological aspects of SARS-CoV-2 infection speculating about their impact on COVID-19 vaccines' mechanisms of action and focused on the management of MS during the COVID pandemic according to the most recent guidelines and recommendations. Finally, the efficacy of COVID-19 and other well-known vaccines against infectious disease in patients with MS on DMDs is discussed.
Figures